Introduction:
T-cell engaging CD3xCD20 bispecific antibodies like glofitamab, mosunetuzumab and epcoritamab are a new group of immunotherapies with promising results in B-cell lymphomas. T-cell engaging antibodies might have milder adverse effect profile compared to chimeric antigen receptor T-cell therapy (CAR-T). However, the duration of response to these new agents is still uncertain and follow-up times for glofitamab and epcoritamab have been short.
We performed extensive follow-up of all patients in Denmark who received CD3xCD20 bispecific antibodies in early pivotal phase 1 / 2 trials to map the response, adverse effects and long-term survival, and baseline correlates with these outcomes.
Methods:
Patients who received T-cell engaging CD3xCD20 bispecific antibodies from 2017 to 2023 as part of a clinical trial were included. The patients' electronic health records were assessed for sex, age, prior therapy, dose received, stage of disease, response according to Lugano criteria, target lesion response, Cytokine Release Syndrome (CRS) and Immune Effector Cell Associated Neurotoxicity (ICANS) events, infections leading to hospitalization, event-free and overall survival. Kaplan-Meier estimates were provided for all time-to-event end points. Hazard ratios with two-sided 95% confidence intervals were calculated from a Cox proportional-hazards model.
Results:
A total of 130 patients with relapsed/refractory B cell lymphoma who received bispecific CD3xCD20 antibodies were analyzed. The median age of patients was 70 years (21 to 87 years) and 58% were male. The median number of prior lines of therapy was 3 and the median Ann Arbor stage was 4. B-symptoms were present in 18% of patients before treatment. The median follow-up time from first treatment was 14.8 months (4 to 74 months). Best overall response rate in all patients was 75% (95% CI, 67-83); 54 patients (45%) had complete response (CR), and 37 patients (31%) had partial response (PR). Among patients with a complete response (CR), the median duration of response was 22.5 months and 29.4 months in patients with DLBCL and follicular lymphoma, respectively.
Among all patients, the most common adverse effects included CRS (max. grade 1 n=45, max. grade 2 n=28, max. grade 3 n=3 max. grade 4-5 n=0), Varicella Zoster infections (n=8), hypogammaglobulinemia (n=14), therapy-related leukemia (n=3) and autoimmune colitis or hepatitis (n=5). Two patients were diagnosed with ICANS, both grade 1. A total of 54 patients were hospitalized due to infections either during or after treatment. The most common infectious agents that lead to hospitalization were Sars-Cov2 (n=20) and Varicella zoster (n=8). Among patients who received the recommended phase two dose (R2PD) the incidence of CRS was 63% while patients who received doses lower than R2PD had an incidence of 53%.
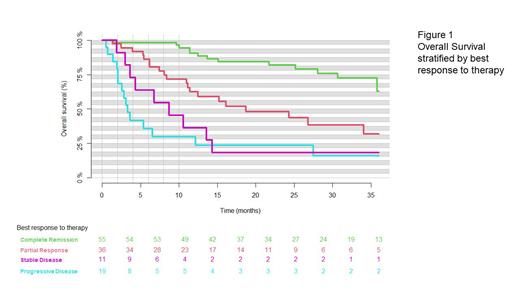
At 12 months after the first treatment, the rate of overall survival (OS) was estimated to 65% in all patients (95% CI, 57-74). (Figure 1), while it was 67% (95% CI, 56-68) in patients who received the recommended phase 2 dose (R2PD). The 24-month overall survival of all patients was estimated at 55 % (95% CI, 45-64), while the 24-month overall survival for patients who had received R2PD was 58% (95% CI, 45-70). The 24-month event free survival of all patients was estimated at 42% (95% CI, 33-51), while the 24-month event free survival for patients who had received the recommended phase 2 dose was 50% (95% CI, 39-62)
Five patients died of disease progression within 30 days after first treatment. No deaths were attributed to bispecific antibodies or CRS. Among patients with a complete response (CR) the rate of PFS at 12 months was 84% (95% CI, 75-95). Of the patients who achieved complete remission, 73% (95% CI, 59-87) were still in complete remission after 36 months (Figure 1).
Conclusions:
We here present long-term national follow-up of heavily pre-treated patients who have received glofitamab or epcoritamab as part of early pivotal phase 1 / 2 trials. We found an overall response rate of 75% in all patients with 45% achieving complete remission. Most common adverse events were CRS and hypogammaglobulinemia, with the incidence of ICANS being notably low. Additionally, we found that CD3xCD20 bispecific antibodies can induce durable long-term responses with 73% of those who achieve complete remission still being in remission after 3 years.
Disclosures
Clausen:AbbVie, Janssen, Gilead, Astra Sencea, Genmab, Roche, Incyte:: Consultancy. Niemann:Carsten Niemann has received research funding and/or consultancy fees from AstraZeneca, Janssen, AbbVie, Beigene, Genmab, CSL Behring, Octapharma, Takeda, and Novo Nordisk Foundation.: Consultancy, Research Funding. Grønbæk:Kirsten Grønbæk received research support from Janssen and is on the advisory board of Nanexa and GSK.: Consultancy, Research Funding. Hutchings:Martin Hutchings has a Consulting or Advisory Role at Takeda, Roche and Genmab and has received Research Funding from Celgene, Genmab, Roche, Takeda and Novartis.: Consultancy; AbbVie, AstraZeneca, Celgene, Genmab, Janssen, Merck, F. Hoffmann-La Roche Ltd, Takeda: Consultancy; AbbVie, AstraZeneca, Bristol Myers-Squibb, Celgene, Genentech, Genmab, Incyte, Janssen, Merck, Novartis, F. Hoffmann-La Roche Ltd, Takeda: Research Funding; AbbVie, AstraZeneca, Celgene, Genmab, Janssen, Merck, F. Hoffmann-La Roche Ltd, Takeda: Honoraria; AbbVie, AstraZeneca, Celgene, Genmab, Janssen, Merck, F. Hoffmann-La Roche Ltd, Takeda: Membership on an entity's Board of Directors or advisory committees.